How Many Electrons Protons and Neutrons Does Lithium Have
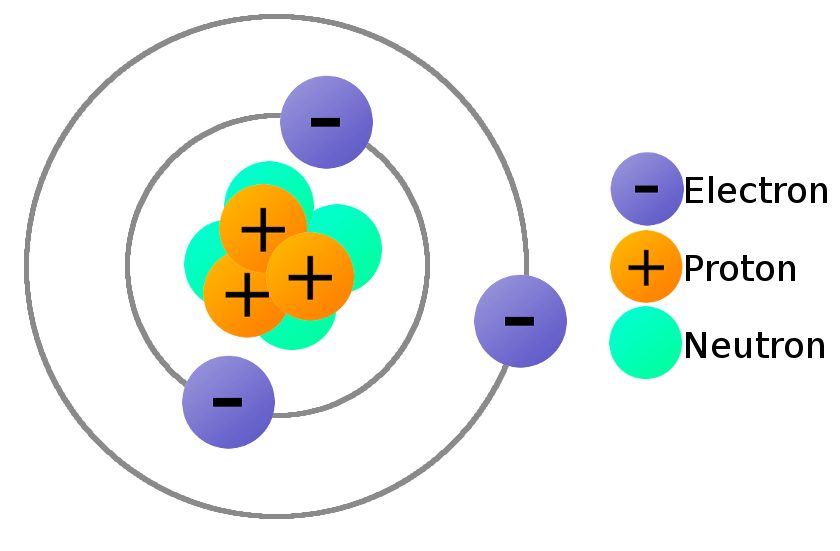
The atomic number is the number of protons. Electrons equal to protons are located in a circular shell outside the nucleus.
Also the number of neutrons would be the difference between the mass number and the atomic number.
. Since Lithium always has 3 protons otherwise it would be a different element it can be deduce that it has only 2 neutrons. 14 How many unpaired electrons does mn2o3 have. If the atom is neutral uncharged it will have 3 electrons.
The mass number of the atom M is equal to the sum of the number of protons and neutrons in the nucleus. The atomic number of lithium is 3. Lithium - Protons - Neutrons - Electrons - Electron Configuration.
That is a lithium atom has a total of three electrons. Also how many protons neutrons and electrons are in the ion 129te2 -. 9 How many protons neutrons and electrons are there in nitrogen ion.
13 How many protons does silicon have. For zinc the atomic weight is 6539 so the mass number is closest to 65. For hydrogen 1008 is closer to 1 than 2 so lets call it 1.
Protons and neutrons are located in the nucleus. Lithium is an alkali metal with the atomic number 3 and an atomic mass of 6941 gmol. 17 How many protons and electrons does 15n have.
How Many Neutrons Does Krypton Have48 neutronsHow many neutrons and electrons are in kryptonKrypton-78 is composed of 36 protons 42 neutrons and 36 electronsDoes krypton have neutronsThe mass number A of a given isotope tells you the number of protons Z which is given by the atomic numb. Thus an atom of Lithium has 3 protons 3 electrons and 4 neutrons following the conventional atomic theory of atomic number being equal to the number of protons as well as electrons. Lithium has 3 protons and electrons in its structure.
Number of Neutrons 65 - 30 35. It also has 4 neutrons. 15 How many paired electrons does magnesium have.
FAQ how many electrons does h have. 13 How many unpaired electrons would you expect on vanadium in v2o3. A lithium atom is an atom of the chemical element lithium.
Admin Send an email November 29 2021. 11 How many protons does nitrogen 16 have. A lithium-7 atom contains three protons four neutrons and three electrons.
And usually unless it. 10 How many protons are in nitrogen-14. Number of Neutrons Mass Number - Number of Protons 1 - 1 0.
Lithium by definition has 3 protons. 12 What atom has exactly 15 protons. Lithium is composed of three electrons bound by the electromagnetic force to a nucleus containing three protons along with either three or four neutrons depending on the isotope held together by the strong force.
Lithium has an atomic number 3 and an atomic mass 7. There are two naturally occurring isotopes of lithium lithium-6 with 3 neutrons and lithium-7 with 4 neutrons. This means that lithium has 3 protons 3 electrons and 4 neutrons.
But since the isotope youre talking about has a 3 charge is has lost 3 negative charges 3 electrons so Al3 has 13 protons 14 neutrons and 10 electrons. Lithium has 3 electrons and therefore 3 protons. How many electrons protons and neutrons does Li have.
That is the number of protons in lithium is three.

What Is Electricity Learn Sparkfun Com

How To Find The Number Of Protons Electrons Neutrons For Lithium Li Youtube
How Many Protons Neutrons And Electrons Does Lithium Have Quora


Belum ada Komentar untuk "How Many Electrons Protons and Neutrons Does Lithium Have"
Posting Komentar